A) 2
B) 4
C) 3
D) 1
Correct Answer: B
Solution :
In diamond each carbon atom is \[s{{p}^{3}}\] hybridized and is linked tetrahedrally to four other carbon atoms, with \[C-C\] bond length of \[1.54\overset{\text{o}}{\mathop{\text{A}}}\,\] and bond angle \[{{109}^{o}}28\]. This result in three dimensional network of strong covalent bond which make diamond extremely hard with very high melting point.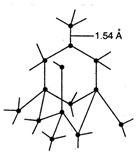
You need to login to perform this action.
You will be redirected in
3 sec
