Direction for: In each of the following questions a statement of Assertion is given followed by a corresponding statement of Reason just below it. Of the statements mark the correct answer as
Assertion: 2-bromobutane on reaction with sodium ethoxide in ethanol gives 1-butene as a major product. Reason: 1-butene is more stable than 2-butene.A) If both assertion and reason are true and reason is not the correct explanation of assertion.
B) If both assertion and reason are true but reason is not the correct explanation of assertion.
C) If assertion is true but reason is false.
D) If both assertion and reason are false.
Correct Answer: D
Solution :
2-bromobutane undergo dehydrohalogenation in presence of strong alkali like sodium ethoxide in ethanol. The elimination is according to Saytzeff rule-"During dehydrohalogenation H is eliminated so as to form more alkylated alkene i.e., from the carbon atom which has less H atoms. \[C{{H}_{3}}C{{H}_{2}}\underset{C{{H}_{3}}}{\mathop{\underset{|}{\mathop{CH}}\,}}\,-Br+Et{{O}^{-}}\xrightarrow[-B{{r}^{-}}]{}\]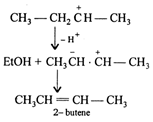 2-butene is more stable than 1-butene due to presence of large number of hyper conjugating structures of 2-butene.
2-butene is more stable than 1-butene due to presence of large number of hyper conjugating structures of 2-butene.
You need to login to perform this action.
You will be redirected in
3 sec
