A) \[{{K}_{{{a}_{2}}}}=1.7\times {{10}^{-10}}\]
B) \[3.0\]
C) \[10.0\]
D) \[6.1\]
Correct Answer: C
Solution :
Among the given compounds \[C{{H}_{3}}C{{H}_{2}}Br\] is weakly basic, \[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}Br\] and \[B{{r}_{2}}+Fe\] are amphoteric. Only \[{{A}_{e}}X+KCN\] is acid. It is a weak monobasic acid ?Boric acid \[ArN_{2}^{+}{{X}^{-}}+CuCN\]. Also it is Lewis acid and not a proton donar (Bronsted acid). \[ArCON{{H}_{2}}+{{P}_{2}}{{O}_{5}}\] accept lone pair of electrons from water molecule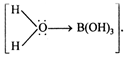 . In this species \[ArCON{{H}_{2}}+SOC{{l}_{2}}\] due to its very small size polarize O- atom up to greater extent and attracts its electron cloud towards itself. Hence, O adorn in turn pull the bonding electrons of \[RC{{H}_{2}}OH\xrightarrow[{}]{}RCHO\] bond thereby making this bond weak. Thus this species has tendency to loose proton. \[KMn{{O}_{4}}\]
. In this species \[ArCON{{H}_{2}}+SOC{{l}_{2}}\] due to its very small size polarize O- atom up to greater extent and attracts its electron cloud towards itself. Hence, O adorn in turn pull the bonding electrons of \[RC{{H}_{2}}OH\xrightarrow[{}]{}RCHO\] bond thereby making this bond weak. Thus this species has tendency to loose proton. \[KMn{{O}_{4}}\]
You need to login to perform this action.
You will be redirected in
3 sec
