A) \[[{{N}_{2}}{{O}_{5}}]\]
B) \[{{[{{N}_{2}}{{O}_{5}}]}_{0}}\]
C) \[{{[{{N}_{2}}{{O}_{5}}]}_{t}}\]
D) \[{{N}_{2}}{{O}_{5}}\]
Correct Answer: D
Solution :
Nickel ions \[P{{H}_{3}},As{{H}_{3}},N{{H}_{3}}\] can be best estimated qualitatively and quantitatively by the use of dimethylglyoxine (DMG) solution. The reaction occurs as follows: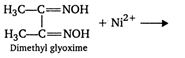
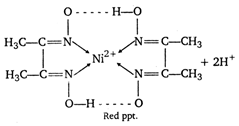 It may be evidently seen that in this reaction each DMG ion lose one proton. Hence, a basic medium is needed to facilitate this reaction. Thus this reaction is carried out in ammonia cal solution \[l=3\]
It may be evidently seen that in this reaction each DMG ion lose one proton. Hence, a basic medium is needed to facilitate this reaction. Thus this reaction is carried out in ammonia cal solution \[l=3\]
You need to login to perform this action.
You will be redirected in
3 sec
