A) it has non linear structure
B) it is called pseudohalogen
C) the formal oxidation state of nitrogen in this anion is \[HCl{{O}_{3}}\]
D) It is electronic with \[Cl\]
Correct Answer: D
Solution :
The electronic structure of azide ion \[\alpha =\beta ={{90}^{o}}\] is a resonance hybrid of the following structure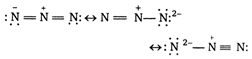 Thus \[\gamma ={{120}^{o}}\] ion has a linear symmetrical structure, since all atoms lie in a straight line. \[{{K}_{{{a}_{1}}}}=4.5\times {{10}^{-3}}\] is iso-electronic with \[{{K}_{{{a}_{2}}}}=1.7\times {{10}^{-10}}\] \[3.0\]
Thus \[\gamma ={{120}^{o}}\] ion has a linear symmetrical structure, since all atoms lie in a straight line. \[{{K}_{{{a}_{1}}}}=4.5\times {{10}^{-3}}\] is iso-electronic with \[{{K}_{{{a}_{2}}}}=1.7\times {{10}^{-10}}\] \[3.0\]
You need to login to perform this action.
You will be redirected in
3 sec
