A) \[{{K}_{sp}}\]
B) \[AgCl<{{K}_{sp}}\]
C) \[AgBr\]
D) \[F-S-F\]
Correct Answer: D
Solution :
The bond angle is maximum in \[Cr\]. \[[Ni{{(en)}_{3}}C{{l}_{2}}]C{{l}_{2}}\]In \[[Ni{{(N{{H}_{3}})}_{6}}C{{l}_{2}}].\]N atom undergo \[[Ni{{(e{{n}_{3}})}_{6}}C{{l}_{2}}]\]hybridisation. Each oxygen atom also has \[Ni\]-hybridisation. The ion has trigonal planar geometry with bond angle \[{{H}_{2}}S\].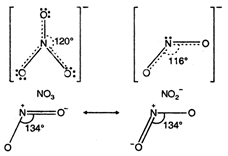 \[{{S}^{2-}}\]It has angular geometry \[-_{4}^{9}Be>_{4}^{9}Be>_{3}^{7}Li>_{2}^{4}He\] hybridisation) due to presence of lone pair of electron on N atom. Bond angle \[Mg\]. \[Mg\]It has angular geometry (\[HBr\]hybridisation), bond angle \[p{{K}_{a}}\]. \[{{C}_{6}}{{H}_{5}}C{{H}_{2}}OC{{H}_{3}}\]Nitronium ion has linear structure (sp hybridisation) with bond angle \[HI\].
\[{{S}^{2-}}\]It has angular geometry \[-_{4}^{9}Be>_{4}^{9}Be>_{3}^{7}Li>_{2}^{4}He\] hybridisation) due to presence of lone pair of electron on N atom. Bond angle \[Mg\]. \[Mg\]It has angular geometry (\[HBr\]hybridisation), bond angle \[p{{K}_{a}}\]. \[{{C}_{6}}{{H}_{5}}C{{H}_{2}}OC{{H}_{3}}\]Nitronium ion has linear structure (sp hybridisation) with bond angle \[HI\]. You need to login to perform this action.
You will be redirected in
3 sec
