A) Electrons flow from copper electrode to zinc electrode
B) Current flows from zinc electrode to copper electrode
C) Cations move toward copper electrode
D) Cations move toward zinc electrode
Correct Answer: C
Solution :
Daniel cell is an electrochemical cell in which \[C{{H}_{3}}NH_{3}^{+}C{{l}^{-}}\] act as cathode and \[NaHS{{O}_{3}}\] act as anode.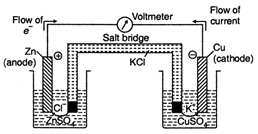 In Daniel cell: (i) Electron flow from the \[{{C}_{6}}{{H}_{5}}NHN{{H}_{2}}\] anode to \[N{{H}_{2}}OH\] cathode and direction of current flow is reverse i.e., \[NaOH-{{I}_{2}}\] (cathode) to \[KOH\] (anode). (ii) The cell reaction is \[C{{H}_{2}}=CHBr\] Thus \[C{{H}_{3}}COC{{H}_{2}}C{{H}_{2}}Br\] ions (cations) move towards \[C{{H}_{3}}C{{H}_{2}}Br\] electrode (cathode) and get accumulated as \[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}Br\] metal.
In Daniel cell: (i) Electron flow from the \[{{C}_{6}}{{H}_{5}}NHN{{H}_{2}}\] anode to \[N{{H}_{2}}OH\] cathode and direction of current flow is reverse i.e., \[NaOH-{{I}_{2}}\] (cathode) to \[KOH\] (anode). (ii) The cell reaction is \[C{{H}_{2}}=CHBr\] Thus \[C{{H}_{3}}COC{{H}_{2}}C{{H}_{2}}Br\] ions (cations) move towards \[C{{H}_{3}}C{{H}_{2}}Br\] electrode (cathode) and get accumulated as \[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}Br\] metal.
You need to login to perform this action.
You will be redirected in
3 sec
