Direction for: In each of the following questions a statement of Assertion is given followed by a corresponding statement of Reason just below it. Of the statements mark the correct answer as
Assertion: Potassium ferrocyanide is diamagnetic, whereas potassium ferricyanide is paramagnetic. Reason: Crystal field splitting in ferrocyanide ion is greater than that of ferricyanide ion.A) If both Assertion and Reason are true and Reason is the correct explanation of Assertion.
B) If both Assertion and Reason are true but Reason is not the correct explanation of Assertion.
C) If Assertion is true but Reason is false.
D) If both Assertion and Reason are false.
Correct Answer: C
Solution :
\[\overset{+2}{\mathop{{{K}_{4}}[Fe{{(CN)}_{6}}]}}\,\]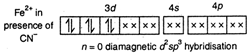 \[\overset{+3}{\mathop{{{K}_{3}}[Fe{{(CN)}_{6}}]}}\,\]
\[\overset{+3}{\mathop{{{K}_{3}}[Fe{{(CN)}_{6}}]}}\,\]  Thus, the assertion that \[{{K}_{4}}[Fe{{(CN)}_{6}}]\]is diamagnetic and \[{{K}_{3}}[Fe{{(CN)}_{6}}]\] is paramagnetic is true. But the reason is false as crystal field splitting in ferrocyanide is less than ferricyanide ion because higher the oxidation state of the metal, greater the crystal field splitting.
Thus, the assertion that \[{{K}_{4}}[Fe{{(CN)}_{6}}]\]is diamagnetic and \[{{K}_{3}}[Fe{{(CN)}_{6}}]\] is paramagnetic is true. But the reason is false as crystal field splitting in ferrocyanide is less than ferricyanide ion because higher the oxidation state of the metal, greater the crystal field splitting.
You need to login to perform this action.
You will be redirected in
3 sec
