A) \[cis-{{[Cr{{({{C}_{2}}{{O}_{4}})}_{2}}C{{l}_{2}}]}^{3-}}\,cis-[Co{{(N{{H}_{3}})}_{4}}C{{l}_{2}}]\]
B) \[[Co{{(en)}_{3}}]C{{l}_{3}},cis-[Co{{(en)}_{2}}C{{l}_{2}}]Cl\]
C) \[[PtCl(diem)]Cl,\,{{[NiC{{l}_{2}}B{{r}_{2}}]}^{2-}}\]
D) \[[Co{{(N{{O}_{3}})}_{3}}{{(N{{H}_{3}})}_{3}}],cis-Pt{{(en)}_{2}}C{{l}_{2}}]\]
Correct Answer: B
Solution :
Both \[cis-[Co{{(en)}_{3}}]C{{l}_{3}}\] and \[cis-[Co{{(en)}_{2}}]Cl\] show optical isomerism.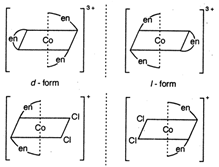 Optical isomers are non-super imposable mirror images of each other. Compounds with co-ordination number 4 and 6 show optical isomerism.
Optical isomers are non-super imposable mirror images of each other. Compounds with co-ordination number 4 and 6 show optical isomerism. | Co-ordination no. | Type |
| 4 | \[M{{(AB)}_{2}}\] |
| 6 | \[[M(AA){{X}_{2}}{{Y}_{2}}],\] \[[M{{(AA)}_{2}}{{X}_{2}})\] or \[[M{{(AA)}_{2}}XY)\] \[[M{{(AA)}_{3}})]\] |
You need to login to perform this action.
You will be redirected in
3 sec
