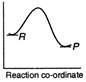
A)
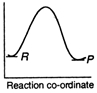
B)
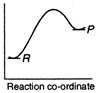
C)
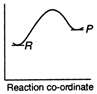
D)
Correct Answer: C
Solution :
Reactions which involve absorption of heat energy are called endothermic reactions. For such reactions \[\Sigma {{H}_{P}}>\Sigma {{H}_{R}}\] In graph (c) and (d) the heat of products is more than heat of reactants \[\therefore \]They represents endothermic reaction. But in (d) only small amount of energy is absorbed (less difference between energy of reactants and products). Thus, (c) represent maximum activation energy. Note: Activation energy is the excess energy that the reactant molecule must possess to cross energy barrier.You need to login to perform this action.
You will be redirected in
3 sec
