Direction for: In each of the following questions a statement of Assertion is given followed by a corresponding statement of Reason just below it. Of the statements mark the correct answer as
Assertion: 2-butenal lacks enolisable H-atom, a to carbonyl group, still it has sufficient acidic character. Reason: The conjugate base of 2-butenal is stabilised by resonance.A) If both Assertion: and Reason: are true and the Reason: is the correct explanation of the Assertion:
B) If both Assertion: and Reason: are true but the Reason: is not the correct explanation of the Assertion:
C) If Assertion: is true but Reason: is false
D) If both Assertion: and Reason: are false
Correct Answer: A
Solution :
2-butenal contains y - H atoms and the conjugate base obtained after the removal of \[\pi \] atom, is stabilised by resonance. Hence, 2-butenal is sufficiently acidic, however, it lacks enolisable H-atom \[\pi \] to carbonyl group.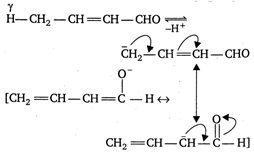
You need to login to perform this action.
You will be redirected in
3 sec
