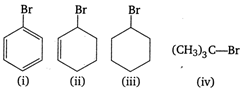
A) \[(i)\,<(iii)\,<(ii)\,<(iv)\]
B) \[N{{O}_{2}}\]
C) \[\pi \]
D) \[\pi \]
Correct Answer: D
Solution :
Cyclic and tertiary halides undergo hydrolysis by \[\therefore \] mechanism and involve the formation of carbocation intermediate. Greater the stability of carbocation intermediate, higher is the ease of halides to undergo hydrolysis. The order of stability of carbocations is as However, C ? Br bond in bromobenzene acquires some double bond character, hence it has least tendency to undergo hydrolysis. Therefore, the increasing order of hydrolysis of the given compounds is
However, C ? Br bond in bromobenzene acquires some double bond character, hence it has least tendency to undergo hydrolysis. Therefore, the increasing order of hydrolysis of the given compounds is 
You need to login to perform this action.
You will be redirected in
3 sec
