A) \[C{{H}_{3}}CH(OH)C{{H}_{3}}\]
B)
C) \[C{{H}_{3}}C{{H}_{2}}CHOHC{{H}_{3}}\]
D) \[(i)<(iii)<(iI)<(iv)\]
Correct Answer: D
Solution :
Compounds having either \[9\times \Delta {{H}_{comb}}(C{{H}_{2}})\]or \[=-210+(9\times -158)\] exhibit geometrical isomerism but the other main condition for exhibiting geometrical isomerism is the presence of two different groups on double bonded carbon atoms. Since in ketoxime; \[=-1632kcal\], two same \[{{V}_{rms}}=\sqrt{\frac{3RT}{M}}=\sqrt{\frac{3\times 8.314\times 298}{4\times {{10}^{-3}}}}=1363m{{s}^{-1}}\] groups are present on double bonded carbon atom, its other form is not possible and it does not exhibit geometrical isomerism. Compounds with \[\lambda =\frac{h}{mv}=\frac{6.626\times {{10}^{-34}}\times 6.023\times {{10}^{23}}}{4\times {{10}^{-3}}\times 1363}\] also have two geometrical forms as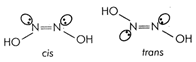
You need to login to perform this action.
You will be redirected in
3 sec
