A) \[(iv)<(ii)<(iii)<(i)\]
B) \[(i)<(iii)<(iv)<(ii)\]
C) \[{{[Cr{{(en)}_{3}}]}^{3+}}\]
D) \[{{[CrC{{l}_{6}}]}^{3-}}\]
Correct Answer: A
Solution :
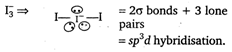

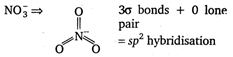
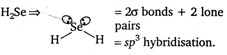 Hence, in 13, d orbitals are used in hybridisation.
Hence, in 13, d orbitals are used in hybridisation.
You need to login to perform this action.
You will be redirected in
3 sec
