A) \[{{[CrC{{l}_{6}}]}^{3-}}\]
B) \[C{{l}^{-}}\]
C) \[U{{F}_{6}}=233+19\times 6=347\]
D) \[\frac{dN}{dt}=kN\]
Correct Answer: B
Solution :
If \[{{n}_{1}}=1\] (heavy water) is taken instead of \[{{n}_{2}}=2\], as solvent, the reaction takes place in the following manner: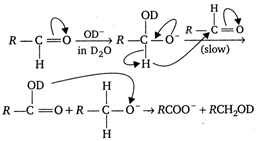
You need to login to perform this action.
You will be redirected in
3 sec
