 The reaction must be
The reaction must be
A) highly spontaneous at ordinary temperature
B) one with negligible enthalpy change
C) endothermic
D) exothermic
Correct Answer: D
Solution :
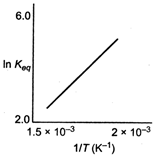
Variation of \[{{K}_{eq}}\] with temperature T is given by vant -Hoff equation. \[\log {{K}_{eq}}=-\frac{\Delta {{H}^{o}}}{2.303RT}+\frac{\Delta {{S}^{o}}}{R}\] Slope of the given line is positive indicating that term A is positive thus \[\Delta {{H}^{o}}\] is negative. Thus, reaction is exothermic.You need to login to perform this action.
You will be redirected in
3 sec
