A) - 0.75, 0.6
B) - 0.75, 1.0
C) - 0.75, 1.25
D) - 3, 1.25
Correct Answer: C
Solution :
Bond order between P?O \[=\frac{\text{no}\text{. of bonds in all possible direction}}{\text{Total no}\text{. of resonating structure}}\] \[=\frac{5}{4}=1.25\] Resonating structures are: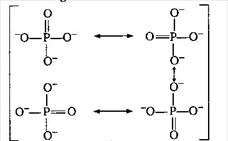 Total charge on \[PO_{4}^{3-}\] ion is \[-3\]. So, the average formal charge on each \['O'\] atom is \[=-\frac{3}{4}=-0.75\]
Total charge on \[PO_{4}^{3-}\] ion is \[-3\]. So, the average formal charge on each \['O'\] atom is \[=-\frac{3}{4}=-0.75\]
You need to login to perform this action.
You will be redirected in
3 sec
