A) \[{{C}_{6}}{{H}_{5}}COO{{C}_{2}}{{H}_{5}}\]
B) \[{{C}_{6}}{{H}_{5}}COO{{C}_{6}}{{H}_{5}}\]
C) \[{{H}_{3}}COC{{H}_{2}}CO{{C}_{6}}{{H}_{5}}\]
D) \[p-{{H}_{3}}CO-{{C}_{6}}{{H}_{4}}-COC{{H}_{3}}\]
Correct Answer: A
Solution :
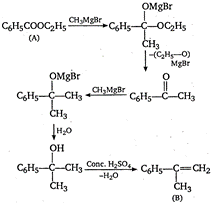 \[{{C}_{6}}{{H}_{5}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,=C{{H}_{2}}\xrightarrow{{{O}_{3}}/{{H}_{2}}O}{{C}_{6}}{{H}_{5}}-\underset{\begin{smallmatrix} | \\ O \\ ({{C}_{8}}{{H}_{8}}O) \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\] \[{{C}_{6}}{{H}_{5}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\xrightarrow[NaOH]{{{I}_{2}}}\,\underset{\text{Iodoform}}{\mathop{CH{{I}_{3}}}}\,+{{C}_{6}}{{H}_{5}}COONa\]
\[{{C}_{6}}{{H}_{5}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{C}}\,=C{{H}_{2}}\xrightarrow{{{O}_{3}}/{{H}_{2}}O}{{C}_{6}}{{H}_{5}}-\underset{\begin{smallmatrix} | \\ O \\ ({{C}_{8}}{{H}_{8}}O) \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\] \[{{C}_{6}}{{H}_{5}}-\underset{\begin{smallmatrix} || \\ O \end{smallmatrix}}{\mathop{C}}\,-C{{H}_{3}}\xrightarrow[NaOH]{{{I}_{2}}}\,\underset{\text{Iodoform}}{\mathop{CH{{I}_{3}}}}\,+{{C}_{6}}{{H}_{5}}COONa\]
You need to login to perform this action.
You will be redirected in
3 sec
