A) phenoxide ion is bulkier than ethoxide
B) phenoxide ion is stronger base than ethoxide
C) phenoxide ion is stabilized through derealization
D) phenoxide ion is less stable than ethoxide
Correct Answer: C
Solution :
Resonance stabilization of phenoxide-ion.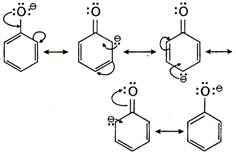 Phenoxide ion is more stable than ethoxies ion due to resonance. Therefore, the ionisation constant of phenoi is higher than ethanol.
Phenoxide ion is more stable than ethoxies ion due to resonance. Therefore, the ionisation constant of phenoi is higher than ethanol.
You need to login to perform this action.
You will be redirected in
3 sec
