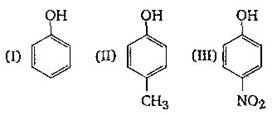
A) I > II > III
B) III > I > II
C) II > III > I
D) I > III > II
Correct Answer: B
Solution :
The acidic behaviour of phenols may be explained due to two reasons? Due to resonance (which is not possible in alcohols), the oxygen atom of the ?OH group acquires a positive charge which helps in the release of a proton.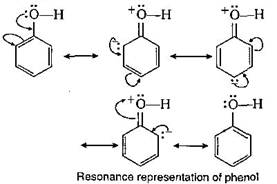 In the dissociation of phenol to phenoxide ion and a proton the equilibrium lies mainly towards the right hand side as the resulting phenoxide ion is more stabilised by resonance as compared to phenol. \[Ar-OH\,\rightleftharpoons \,Ar{{O}^{-}}\,+{{H}^{+}}\]
In the dissociation of phenol to phenoxide ion and a proton the equilibrium lies mainly towards the right hand side as the resulting phenoxide ion is more stabilised by resonance as compared to phenol. \[Ar-OH\,\rightleftharpoons \,Ar{{O}^{-}}\,+{{H}^{+}}\] 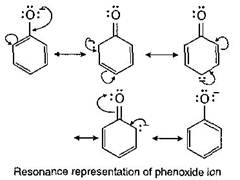 The acidic strength of phenols depends on the nature of substituents present in the benzene nucleus. Electron withdrawing groups like etc. when present in the ortho and para positions with respecophenolic group increases the acidity of phenol due to greater stabilization of phenoxide ion. While the presence of electron releasing group like etc. decrease the acidity of phenols. This explains the following order of acidity. p-nitrophenol > phenol > p-cresol.
The acidic strength of phenols depends on the nature of substituents present in the benzene nucleus. Electron withdrawing groups like etc. when present in the ortho and para positions with respecophenolic group increases the acidity of phenol due to greater stabilization of phenoxide ion. While the presence of electron releasing group like etc. decrease the acidity of phenols. This explains the following order of acidity. p-nitrophenol > phenol > p-cresol.
You need to login to perform this action.
You will be redirected in
3 sec
