A) \[Ni{{(CO)}_{4}}\]-Tetrahedral, paramagnetic
B) \[Ni(CN)_{4}^{2-}\]-Square planar, diamagnetic
C) \[Ni{{(CO)}_{4}}\]-Tetrahedral, diamagnetic
D) \[{{[Ni{{(Cl)}_{4}}]}^{2-}}\] Tetrahedral, paramagnetic
Correct Answer: A
Solution :
In \[Ni{{(CO)}_{4}},\,Ni\] has zero oxidation number So \[_{28}Ni\,=1{{s}^{2}},\,2{{s}^{2}}\,2{{p}^{6}},\,3{{s}^{2}}3{{p}^{6}}3{{d}^{8}},\,4{{s}^{2}}\] In excited state and during the formation of \[Ni{{(CO)}_{4}}\] \[\to \]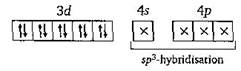 Hence in it, no unpaired electron is present. So it shows the property of diamagnetism and tetrahedral structure.
Hence in it, no unpaired electron is present. So it shows the property of diamagnetism and tetrahedral structure.
You need to login to perform this action.
You will be redirected in
3 sec
