A) \[\pi \]-molecular orbital
B) \[\sigma \]-molecular orbital
C) \[\delta \]-molecular orbital
D) No bond will form
Correct Answer: A
Solution :
For \[\pi \] -overlap the lobes of the atomic orbitals are perpendicular to the line joining the nuclei.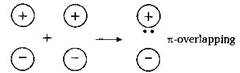
You need to login to perform this action.
You will be redirected in
3 sec
