A) 1
B) 2
C) 3
D) 4
Correct Answer: A
Solution :
n = 3 \[l=2\] m = + 2 \[s=\,\,\pm \,1/2\] These values of quantum numbers are possible for an orbital of 3d sub-shell as the possible value of m for \[l=2\] is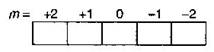
You need to login to perform this action.
You will be redirected in
3 sec
