A) \[NO_{3}^{-}\]
B) \[SO_{3}^{2-}\]
C) \[BO_{3}^{3-}\]
D) \[CO_{3}^{2-}\]
Correct Answer: B
Solution :
In \[SO_{3}^{2-}\] the S is sp3 hybridised, so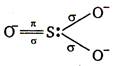 \[\underset{\begin{smallmatrix} \text{(Sulphur atom in} \\ \text{ excited state)} \end{smallmatrix}}{\mathop{_{16}S=1{{s}^{2}},\,2{{s}^{2}}\,2{{p}^{6}}}}\,,\,\underbrace{3{{s}^{2}}3p_{x}^{1}3p_{y}^{1}3p_{z}^{1}}_{s{{p}^{3}}\,\text{hybridisation}}\,\underset{\text{unhybridised}}{\mathop{3d_{xy}^{1}}}\,\] In 'S' the three p-orbitals forms \[\sigma \] bonds with three oxygen atoms and unhybridised d-orbital is involved in \[\pi \] bond formation \[_{16}O=\,1{{s}^{2}},2\,{{s}^{2}}\,2p_{x}^{2}\,2p_{y}^{1}\,2p_{z}^{1}\] In oxygen two unpaired p-orbitals are present, one is involved in \[\sigma \] bond formation while other is used in \[\pi \] bond formation. Thus in \[SO_{3}^{2-}\] \[{{p}_{\pi }}\] and \[{{d}_{\pi }}\] orbitals are involved for \[{{p}_{\pi }}-{{d}_{\pi }}\] bonding.
\[\underset{\begin{smallmatrix} \text{(Sulphur atom in} \\ \text{ excited state)} \end{smallmatrix}}{\mathop{_{16}S=1{{s}^{2}},\,2{{s}^{2}}\,2{{p}^{6}}}}\,,\,\underbrace{3{{s}^{2}}3p_{x}^{1}3p_{y}^{1}3p_{z}^{1}}_{s{{p}^{3}}\,\text{hybridisation}}\,\underset{\text{unhybridised}}{\mathop{3d_{xy}^{1}}}\,\] In 'S' the three p-orbitals forms \[\sigma \] bonds with three oxygen atoms and unhybridised d-orbital is involved in \[\pi \] bond formation \[_{16}O=\,1{{s}^{2}},2\,{{s}^{2}}\,2p_{x}^{2}\,2p_{y}^{1}\,2p_{z}^{1}\] In oxygen two unpaired p-orbitals are present, one is involved in \[\sigma \] bond formation while other is used in \[\pi \] bond formation. Thus in \[SO_{3}^{2-}\] \[{{p}_{\pi }}\] and \[{{d}_{\pi }}\] orbitals are involved for \[{{p}_{\pi }}-{{d}_{\pi }}\] bonding.
You need to login to perform this action.
You will be redirected in
3 sec
