A) \[[Cr{{(CO)}_{6}}]\]
B) \[[Fe{{(CO)}_{5}}]\]
C) \[{{[Fe{{(CN)}_{6}}]}^{4-}}\]
D) \[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
Correct Answer: D
Solution :
Atoms, ions or molecules having unpaired electrons are paramagnetic. In \[[Cr(N{{H}_{3}})_{6}^{3+}\]Cr is present as Cr (III) or \[C{{r}^{3+}}\] So electronic configuration is \[\underset{Ground\,state}{\mathop{_{24}Cr+}}\,1{{s}^{2}},\,2{{s}^{2}}\,2{{p}^{6}},\,3{{s}^{2}}\,3{{p}^{6}}\,3{{d}^{5}},\,4{{s}^{1}}\] \[C{{r}^{3+}}=1{{s}^{2}},\,2{{s}^{2}}2{{p}^{6}},\,3{{s}^{2}}3{{p}^{6}}3{{d}^{3}}\]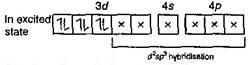 Number of unpaired electrons = 3 \[In\,[Cr{{(CO)}_{6}}](O.\,N.\,of\,Cr=0)\] \[\underset{(Ground\,state)}{\mathop{{{\,}_{24}}Cr}}\,=1{{s}^{2}},\,2{{s}^{2}}2{{p}^{6}},\,3{{s}^{2}}3{{p}^{6}}3{{d}^{5}},\,4{{s}^{1}}\]
Number of unpaired electrons = 3 \[In\,[Cr{{(CO)}_{6}}](O.\,N.\,of\,Cr=0)\] \[\underset{(Ground\,state)}{\mathop{{{\,}_{24}}Cr}}\,=1{{s}^{2}},\,2{{s}^{2}}2{{p}^{6}},\,3{{s}^{2}}3{{p}^{6}}3{{d}^{5}},\,4{{s}^{1}}\] 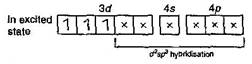 Number of unpaired electron = 0 \[In\,[Fe{{(CO)}_{5}}]\,\,(ON\,of\,Fe=0)\] \[\,\underset{(Ground\,state)}{\mathop{_{26}Fe}}\,=1{{s}^{2}},\,2{{s}^{2}}\,2{{p}^{6}},\,3{{s}^{2}}\,3{{p}^{6}}\,3{{d}^{6}},4{{s}^{2}}\]
Number of unpaired electron = 0 \[In\,[Fe{{(CO)}_{5}}]\,\,(ON\,of\,Fe=0)\] \[\,\underset{(Ground\,state)}{\mathop{_{26}Fe}}\,=1{{s}^{2}},\,2{{s}^{2}}\,2{{p}^{6}},\,3{{s}^{2}}\,3{{p}^{6}}\,3{{d}^{6}},4{{s}^{2}}\] 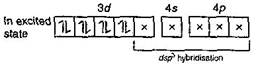 Number of unpaired electron = 0 In \[{{[Fe{{(CN)}_{6}}]}^{4-}}\,(O\,No\,of\,Fe=+2)\] \[\underset{(Ground\,state)}{\mathop{F{{e}^{2+}}}}\,=1{{s}^{2}},\,2{{s}^{2}}\,2{{p}^{6}},\,3{{s}^{2}}\,3{{p}^{6}}\,3{{d}^{6}}\]
Number of unpaired electron = 0 In \[{{[Fe{{(CN)}_{6}}]}^{4-}}\,(O\,No\,of\,Fe=+2)\] \[\underset{(Ground\,state)}{\mathop{F{{e}^{2+}}}}\,=1{{s}^{2}},\,2{{s}^{2}}\,2{{p}^{6}},\,3{{s}^{2}}\,3{{p}^{6}}\,3{{d}^{6}}\] 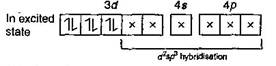 Number of unpaired electron = 0 Hence, in above complex ion paramagnetic character is in \[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}\] as it contains three unpaired electrons.
Number of unpaired electron = 0 Hence, in above complex ion paramagnetic character is in \[{{[Cr{{(N{{H}_{3}})}_{6}}]}^{3+}}\] as it contains three unpaired electrons.
You need to login to perform this action.
You will be redirected in
3 sec
