A) 50% D + 50% L-isomer
B) 20% D + 80% L-isomer
C) D-isomer
D) L-isomer
Correct Answer: A
Solution :
Lactice acid obtained in the given reaction is an optically active compound due to the presence of chiral C-atom. It exits as d and \[l\] forms whose ratio is 1 : 1.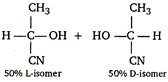
You need to login to perform this action.
You will be redirected in
3 sec
