A) 4
B) zero
C) 2
D) 3 (Atomic number Co = 27)
Correct Answer: A
Solution :
In complex ion \[{{[Co{{F}_{6}}]}^{3-}}\,,\,Co\] is present at +3 oxidation state \[\begin{align} & _{27}Co=1{{s}^{2}},2{{s}^{2}}\,2{{p}^{6}},\,3{{s}^{2}}3{{p}^{6}}\,3{{d}^{7}},4{{s}^{2}} \\ & C{{o}^{3+}}=1{{s}^{2}},2{{s}^{2}}\,2{{p}^{6}},3{{s}^{2}}3{{p}^{6}}\,3{{d}^{6}} \\ \end{align}\]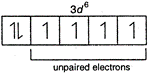 Thus, the number of unpaired electrons in 3d sub-shell of \[{{[Co{{F}_{6}}]}^{3-}}\] is 4
Thus, the number of unpaired electrons in 3d sub-shell of \[{{[Co{{F}_{6}}]}^{3-}}\] is 4
You need to login to perform this action.
You will be redirected in
3 sec
