A) \[a\ne b\ne c\] and \[\alpha \ne \beta \] and \[\gamma \ne {{90}^{o}}\]
B) \[a\ne b\ne c\] and \[\alpha =\beta =\gamma ={{90}^{o}}\]
C) \[a=b=c\] and \[\alpha \ne \beta \ne \gamma ={{90}^{o}}\]
D) \[a=b=c\] and \[\alpha =\beta =\gamma ={{90}^{o}}\]
Correct Answer: D
Solution :
In cubic crystals, the crystal axes are perpendicular to one another \[(\alpha =\beta =\gamma ={{90}^{o}})\] and the repetitive interval is the same along the three axes \[(a=b=c)\].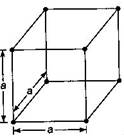 Note: Cubic crystal may be simple, body centred or face centred.
Note: Cubic crystal may be simple, body centred or face centred.
You need to login to perform this action.
You will be redirected in
3 sec
