A) CO2 < \[CO_{3}^{2-}\] < CO
B) CO < \[CO_{3}^{2-}\]< CO2
C) \[CO_{3}^{2-}\]< CO2 < CO
D) CO < CI2 < \[CO_{3}^{2-}\]
Correct Answer: D
Solution :
A bond length is the average distance between the centres of nuclei of two bonded atoms. A multiple bond (double or triple bond) is always shorter than die corresponding single bond.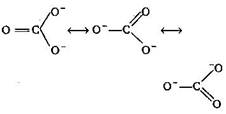 The C-atom in \[CO_{3}^{2-}\]is sp2 hybridized as shown The C-atom in \[C{{O}_{2}}\] is sp hybridized with bond distance of carbon-oxygen is 122 pm. \[\begin{align} & O==C==O\overset{{}}{\longleftrightarrow}{{\,}^{+}}O\,\equiv \equiv \,C\equiv \equiv C-\bar{O}\overset{{}}{\longleftrightarrow} \\ & \bar{O}--C\equiv \equiv \overset{+}{\mathop{O}}\, \\ \end{align}\] The C-atom in CO is sp hybridized with C?O bond distance is 110 pm : C \[\equiv \] O+ : So the correct order is \[CO<C{{O}_{2}}<CO_{3}^{2-}\]
The C-atom in \[CO_{3}^{2-}\]is sp2 hybridized as shown The C-atom in \[C{{O}_{2}}\] is sp hybridized with bond distance of carbon-oxygen is 122 pm. \[\begin{align} & O==C==O\overset{{}}{\longleftrightarrow}{{\,}^{+}}O\,\equiv \equiv \,C\equiv \equiv C-\bar{O}\overset{{}}{\longleftrightarrow} \\ & \bar{O}--C\equiv \equiv \overset{+}{\mathop{O}}\, \\ \end{align}\] The C-atom in CO is sp hybridized with C?O bond distance is 110 pm : C \[\equiv \] O+ : So the correct order is \[CO<C{{O}_{2}}<CO_{3}^{2-}\]
You need to login to perform this action.
You will be redirected in
3 sec
