A) \[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{2}}-C{{H}_{2}}Br\]
B) \[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\overset{\begin{smallmatrix} Br \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{2}}C{{H}_{3}}\]
C) \[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ Br \end{smallmatrix}}{\mathop{CH}}\,-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}\]
D) \[C{{H}_{3}}-\underset{\begin{smallmatrix} | \\ C{{H}_{3}} \end{smallmatrix}}{\mathop{CH}}\,-\underset{\begin{smallmatrix} | \\ Br \end{smallmatrix}}{\mathop{CH}}\,-C{{H}_{3}}\]
Correct Answer: B
Solution :
Key Idea: The mechanism of electrophilic addition reaction is consistent with the occurence of rearrangement leading to more stable carbocation. The order of stability of carbocation is as: \[T>S>P>C{{H}_{3}}\] \[{{H}_{3}}C-\underset{\begin{smallmatrix} | \\ H \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-CH=C{{H}_{2}}+HBr\xrightarrow[-Br]{{}}\]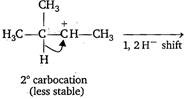 \[{{H}_{3}}C-\underset{\begin{smallmatrix} {{3}^{o}}\,carbocation \\ (more\,stable) \end{smallmatrix}}{\mathop{\underset{\oplus }{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-}}\,C{{H}_{2}}-C{{H}_{3}}\xrightarrow[{}]{B{{r}^{-}}}\] \[{{H}_{3}}C-\underset{\begin{smallmatrix} | \\ Br \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{2}}-C{{H}_{3}}\]
\[{{H}_{3}}C-\underset{\begin{smallmatrix} {{3}^{o}}\,carbocation \\ (more\,stable) \end{smallmatrix}}{\mathop{\underset{\oplus }{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-}}\,C{{H}_{2}}-C{{H}_{3}}\xrightarrow[{}]{B{{r}^{-}}}\] \[{{H}_{3}}C-\underset{\begin{smallmatrix} | \\ Br \end{smallmatrix}}{\overset{\begin{smallmatrix} C{{H}_{3}} \\ | \end{smallmatrix}}{\mathop{C}}}\,-C{{H}_{2}}-C{{H}_{3}}\]
You need to login to perform this action.
You will be redirected in
3 sec
