A) \[sp,\,s{{p}^{3}},\,s{{p}^{2}}\] and \[s{{p}^{3}}\]
B) \[s{{p}^{3}},\,s{{p}^{2}},\,s{{p}^{2}}\] and \[sp\]
C) \[sp,\,s{{p}^{2}},\,s{{p}^{2}}\] and \[s{{p}^{3}}\]
D) \[sp,\,s{{p}^{2}},\,s{{p}^{3}}\] and \[s{{p}^{2}}\]
Correct Answer: A
Solution :
Key Idea Count number of a bond and then find hybridisation as follows. If number of \[1.3\times {{10}^{4}}g\] bonds =2; hybridisation is sp, If number of \[CC{{l}_{3}}CHO\]bonds = 3; hybridisation is \[(At.no.Zn=30,Sc=21,Ti=22,Cr=24)\] If number of\[{{[Sc{{({{H}_{2}}O)}_{3}}{{(N{{H}_{3}})}_{3}}]}^{3+}}\] bonds = 4; hybridisation is \[{{[Ti{{(en)}_{2}}{{(N{{H}_{3}})}_{2}}]}^{4+}}\]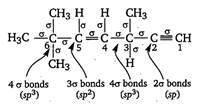 Double and triple bonds are not considered while finding hybridisation.
Double and triple bonds are not considered while finding hybridisation.
You need to login to perform this action.
You will be redirected in
3 sec
