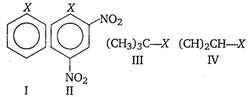
A) I < II < IV < III
B) II < III < I < IV
C) IV < III < I < II
D) III < II < I < IV
Correct Answer: A
Solution :
Key Idea Alkyl halides are more reactive towards nucleophilic substitution. Reactivity depends upon the stability of carbocation intermediate formed. Among the given halides, aryl halide \[({{C}_{6}}{{H}_{5}}X)\]is least reactive towards nucleophile as in it the C - X bond acquire some double bond character due to resonance. Presence of electron withdrawing groups like ?\[\text{N}{{\text{O}}_{\text{2}}}\] at ortho and para positions facilitate the nucleophilic displacenent of ?X of aryl halide. Among alkyl halides, 3° halides are more reactive as compared to 2° halides due to the formation of more stable carbocation. Hence, the order of reactivity of C ? X bond towards nucleophile is as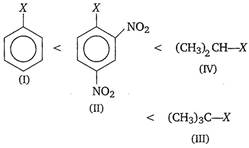
You need to login to perform this action.
You will be redirected in
3 sec
