A) \[{{[Ni{{(CN)}_{4}}]}^{2-}}\]
B) \[{{[CuC{{l}_{4}}]}^{2-}}\]
C) \[{{[Co{{F}_{6}}]}^{3-}}\]
D) \[{{[NiC{{l}_{4}}]}^{2-}}\]
Correct Answer: A
Solution :
Electronic configuration of \[N{{i}^{2+}}\] in \[{{[Ni{{(CN)}_{4}}]}^{2-}}\] is \[N{{i}^{2+}}=3{{d}^{8}}4{{s}^{o}}\]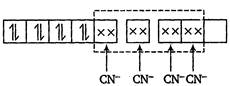 \[\therefore {{[Ni{{(CN)}_{4}}]}^{2-}}\]is diamagnetic (because of the absence of unpaired electrons).
\[\therefore {{[Ni{{(CN)}_{4}}]}^{2-}}\]is diamagnetic (because of the absence of unpaired electrons).
You need to login to perform this action.
You will be redirected in
3 sec
