A) B > A > D > E
B) B > D > C > A
C) A > B > C > D
D) A > C > B > D
Correct Answer: C
Solution :
If an electron withdrawing group (\[-I\] showing group) is present, it makes the removal of proton more easy by stabilizing the remaining carboxylate ion and thus, makes the acid more acidic. The order of acidity of given compounds is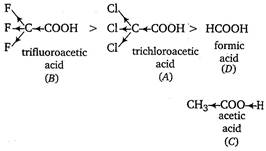
You need to login to perform this action.
You will be redirected in
3 sec
