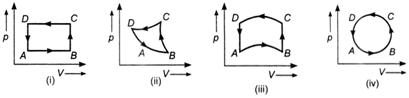
 The change in internal energy of the gas will be
The change in internal energy of the gas will be
A) positive in all the cases (i) to (iv)
B) positive in cases (i), (ii) and (iii) but zero in case (iv)
C) negative in cases (i), (ii) and (iii) but zero in case (iv)
D) zero in all the four cases
Correct Answer: D
Solution :
Internal energy of a thermodynamic system is a characteristic property of the state of the system, it does not matter how that state has been obtained. If a system starting from one state passes through different states and finally returns to its initial state, then the change in its internal energy will be zero.You need to login to perform this action.
You will be redirected in
3 sec
