A) \[C{{H}_{3}}.\,C{{H}_{2}}COOH\]
B) \[HOOCC{{H}_{2}}COOH\]
C) \[C{{H}_{2}}COOH\]
D) \[{{C}_{6}}{{H}_{5}}COOH\]
Correct Answer: B
Solution :
Key Idea Decarboxylation-The removal of carbon dioxide from carboxylic group is called decarboxylation.) When two carboxylic groups are attached to same carbon atom, decarboxylation takes place easily. In malonic acid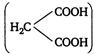 carboxylic groups are attached to the same carbon atom, hence, it undergoes easy thermal decomposition.
carboxylic groups are attached to the same carbon atom, hence, it undergoes easy thermal decomposition. You need to login to perform this action.
You will be redirected in
3 sec
