A) \[[Fe{{(CN)}_{6}}{{[}^{4-}}\]
B) \[{{[Cu{{({{H}_{2}}O)}_{4}}]}^{2+}}\]
C) \[{{[Zn{{(N{{H}_{3}})}_{4}}]}^{2+}}\]
D) \[{{[Mn{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
Correct Answer: D
Solution :
Key Idea Paramagnetic character \[\propto \] number of unpaired electrons \[{{[Fe{{(CN)}_{6}}]}^{4-}}\] Iron is in +2 oxidation state.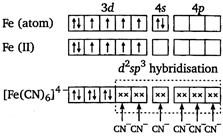 There is no unpaired electron in \[[Fe{{(C{{N}_{6}}]}^{4-}}\]. So, it is diamagnetic. \[{{[Cu{{({{H}_{2}}O)}_{4}}]}^{2+}}\] Copper is in +2 oxidation state.
There is no unpaired electron in \[[Fe{{(C{{N}_{6}}]}^{4-}}\]. So, it is diamagnetic. \[{{[Cu{{({{H}_{2}}O)}_{4}}]}^{2+}}\] Copper is in +2 oxidation state. 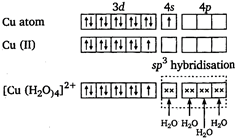 There is one unpaired electron. So, it is paramagnetic. \[{{[Zn{{(N{{H}_{3}})}_{4}}]}^{2+}}\] zinc is in +2 oxidation state.
There is one unpaired electron. So, it is paramagnetic. \[{{[Zn{{(N{{H}_{3}})}_{4}}]}^{2+}}\] zinc is in +2 oxidation state. 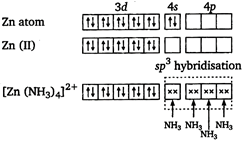 There is no unpaired electron. So, it is diamagnetic. \[{{[Zn{{(N{{H}_{3}})}_{4}}]}^{2+}}\] Mn is in +2 oxidation state.
There is no unpaired electron. So, it is diamagnetic. \[{{[Zn{{(N{{H}_{3}})}_{4}}]}^{2+}}\] Mn is in +2 oxidation state. 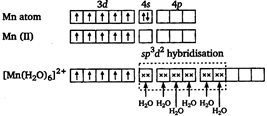 In \[{{[Mn{{({{H}_{2}}O)}_{6}}]}^{2+}}\] there are five unpaired electrons. So, it has maximum paramagnetic character.
In \[{{[Mn{{({{H}_{2}}O)}_{6}}]}^{2+}}\] there are five unpaired electrons. So, it has maximum paramagnetic character.
You need to login to perform this action.
You will be redirected in
3 sec
