A)
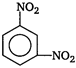
B)

C)
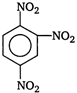
D)
![]()
Correct Answer: A
Solution :
Key Idea In nitrobenzene \[N{{O}_{2}}\]group is present which electron withdrawing group is. So, the electron density is reduced at o- and p-positions. The electron density at m-position is more than o- and p-positions. So, nitro group is meta directing. Moreover ?\[N{{O}_{2}}\]group is electron withdrawing group and it deactivates the benzene ring. Therefore, nitrobenzene is less reactive towards electrophilic substitution reaction and it undergoes reactions only under drastic conditions. Nitrobenzene on reaction with fuming \[HN{{O}_{3}}\], at \[{{90}^{o}}C\], gives m-dinitrobenzene.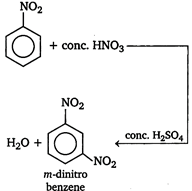
You need to login to perform this action.
You will be redirected in
3 sec
