A) a monobasic acid
B) atribasicacid
C) a dibasic acid
D) not acidic at all
Correct Answer: A
Solution :
Hypophosphorus acid \[{{H}_{3}}P{{O}_{2}}\] is a monobasic acid because it forms only one type of salts eg, sodium hydrogen phosphite \[(Na{{H}_{2}}P{{O}_{2}})\] \[NaOH+{{H}_{3}}P{{O}_{2}}\xrightarrow{{}}Na{{H}_{2}}P{{O}_{2}}+{{H}_{2}}O\] The structure of \[{{H}_{3}}P{{O}_{2}}\] is following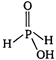 In the structure of hypophosphorus acid two hydrogen atom is attached to phosphorus (These two hydrogen atoms are non ionisable) and one hydrogen atom is attached to oxygen atom and this hydrogen atom is ionisable. Therefore, hypophosphorus acid forms only one type of salt and due to presence of only one ionisable hydrogen atom it is a monobasic acid. \[{{H}_{3}}P{{O}_{2}}{{H}_{2}}PO_{2}^{-}+{{H}^{+}}\]
In the structure of hypophosphorus acid two hydrogen atom is attached to phosphorus (These two hydrogen atoms are non ionisable) and one hydrogen atom is attached to oxygen atom and this hydrogen atom is ionisable. Therefore, hypophosphorus acid forms only one type of salt and due to presence of only one ionisable hydrogen atom it is a monobasic acid. \[{{H}_{3}}P{{O}_{2}}{{H}_{2}}PO_{2}^{-}+{{H}^{+}}\]
You need to login to perform this action.
You will be redirected in
3 sec
