A) Phenol
B) Hydrazobenzene
C) Aniline
D) Nitrosobenzene
Correct Answer: B
Solution :
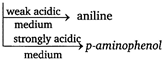
Key Idea Reduction of nitrobenzene| Medium | Reagent | Product |
| Acid | Sn/HCl | Aniline |
| Neutral | \[Zn/N{{H}_{4}}Cl\] | N-phenyl hydroxyl amine |
| Alkaline | \[N{{a}_{3}}As{{O}_{3}}\]/NaOH, \[C{{H}_{3}}OH\]Zn/NaOH,\[{{C}_{2}}{{H}_{5}}OH\] | Azoxy benzene Hydrazo benzene |
| Metallic | \[LiAl{{H}_{4}}\] | Aniline |
| Hydride | ||
| Electrolytic | Dil \[{{H}_{2}}S{{O}_{4}}\] | Aniline |
| Reduction |
 When nitrobenzene is reduced by zinc and alkali then hydrazobenzene is formed.
When nitrobenzene is reduced by zinc and alkali then hydrazobenzene is formed. 
You need to login to perform this action.
You will be redirected in
3 sec
