A) nitrogen atom is smaller than boron atoms
B) B?Cl bond is more polar than N?Cl bond
C) N?Cl bond is more covalent than B?Cl bond
D) \[FC{{l}_{3}}\] has no lone pair but \[NC{{l}_{3}}\] has a lone pair of electrons
Correct Answer: D
Solution :
In boron trichloride, boron is \[s{{p}^{2}}\] hybridised and there is no lone pair on boron hence, \[BC{{l}_{3}}\] is planar in shape while in \[NC{{l}_{3}}\] the nitrogen is \[s{{p}^{3}}\] hybridised and there is a lone pair on nitrogen atom hence, the shape of \[NC{{l}_{3}}\] is pyramidal.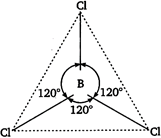 Trigonal planar geometry of \[BC{{l}_{3}}\] molecule
Trigonal planar geometry of \[BC{{l}_{3}}\] molecule 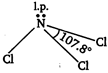 Pyramidal shape of \[NC{{l}_{3}}\] is molecule
Pyramidal shape of \[NC{{l}_{3}}\] is molecule
You need to login to perform this action.
You will be redirected in
3 sec
