A) \[{{[Co{{(N{{H}_{3}})}_{6}}]}^{2+}}\] is oxidised to diamagnetic \[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\] by the oxygen in air
B) Tetrahedral complexes are more stable than octahedral complexes
C) \[{{[Fe{{(CN)}_{6}}]}^{3-}}\] is stable but \[{{[Fe{{F}_{6}}]}^{3-}}\] is unstable
D) The \[{{[Cu{{[N{{H}_{3}})}_{4}}]}^{2+}}\] ion has a tetrahedral geometry and is diamagnetic
Correct Answer: A
Solution :
\[{{[Co{{(N{{H}_{3}})}_{6}}]}^{2+}}=[Ar]\]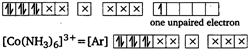 (No unpaired electron, so diamagnetic) Tetrahedral complexes are less stable than octahedral complexes. \[{{[Fe{{(CN)}_{6}}]}^{3-}}\] and \[{{[Fe{{F}_{6}}]}^{3-}}\] both are stable but former is more stable. \[{{[Cu{{(N{{H}_{3}})}_{4}}]}^{2+}}\] has square planar geometry and is paramagnetic.
(No unpaired electron, so diamagnetic) Tetrahedral complexes are less stable than octahedral complexes. \[{{[Fe{{(CN)}_{6}}]}^{3-}}\] and \[{{[Fe{{F}_{6}}]}^{3-}}\] both are stable but former is more stable. \[{{[Cu{{(N{{H}_{3}})}_{4}}]}^{2+}}\] has square planar geometry and is paramagnetic.
You need to login to perform this action.
You will be redirected in
3 sec
