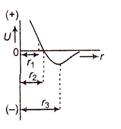
 Read the following statements carefully The equilibrium separation distance between the atoms is equal to \[{{r}_{2}}\] At \[\text{r}={{\text{r}}_{\text{1}}},\]the force between the atoms is repulsive For \[\begin{align} & \text{V}{{\text{p}}^{\text{2}}}= \\ & \\ \end{align}\] the force between the atoms is attractive TO. Which of the above statements is true?
Read the following statements carefully The equilibrium separation distance between the atoms is equal to \[{{r}_{2}}\] At \[\text{r}={{\text{r}}_{\text{1}}},\]the force between the atoms is repulsive For \[\begin{align} & \text{V}{{\text{p}}^{\text{2}}}= \\ & \\ \end{align}\] the force between the atoms is attractive TO. Which of the above statements is true?
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
Correct Answer: A
Solution :
Its the distance decreases attraction increase. So the equilibrium separation distance between the atoms is equal to\[{{r}_{2}}\].You need to login to perform this action.
You will be redirected in
3 sec
