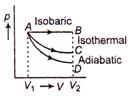
A) \[{{U}_{B}}-{{U}_{A}}=0\]
B) \[{{U}_{C}}-{{U}_{A}}=0\]
C) \[{{U}_{D}}-{{U}_{A}}<0\]
D) \[{{U}_{B}}-{{U}_{C}}<0\]
Correct Answer: D
Solution :
In isothermal process \[{{U}_{C}}-{{U}_{A}}=0\] In isobaric process (p = constant) Hence, temperature of gas will increase \[{{U}_{B}}-{{U}_{A}}>0\] In adiabatic process Q =constant. Hence temperature will decrease \[{{U}_{D}}-{{U}_{A}}<0\] So, \[{{U}_{B}}={{U}_{C}}={{U}_{D}}\] is not correct.You need to login to perform this action.
You will be redirected in
3 sec
