A) \[s{{p}^{2}}\]-hybrid orbitals for bonding
B) p-orbitals for bonding
C) \[s{{p}^{3}}\]-hybrid orbitals for bonding
D) \[ds{{p}^{2}}\] hybrid for bonding
Correct Answer: A
Solution :
In diborane, boron atom is \[s{{p}^{2}}\]-hybridised with bond angle of \[{{120}^{o}}\] and having triangular planar structure.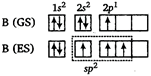 They form \[\sigma \]-bonds with H-atom by \[(s{{p}^{2}}-s)\] overlapping.
They form \[\sigma \]-bonds with H-atom by \[(s{{p}^{2}}-s)\] overlapping.
You need to login to perform this action.
You will be redirected in
3 sec
