A) Tetrahedral, Paramagnetic Tetrahedral, Diamagnetic
B) Tetrahedral, Diamagnetic Square planar, Diamagnetic
C) Square planar, Diamagnetic Square planar, Paramagnetic
D) Trigonal pyramidal Square pyramidal
Correct Answer: B
Solution :
(I) In \[[Ni{{(CO)}_{4}}]\], Ni is present as Ni atom. Since, CO is a strong field ligand, therefore, it pairs up the unpaired electrons. \[_{28}Ni=[Ar]\,3{{d}^{8}}\,\,4{{s}^{2}}\]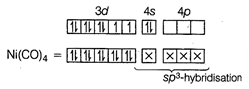 Due to \[S{{p}^{3}}\]-hybridisation, its shape is tetrahedral and due to absence of unpaired electron, it is diamagnetic in nature. II. In \[{{[Ni{{(CN)}_{4}}]}^{2-}}\], Ni is present as \[N{{i}^{2+}}\]-ion, since \[C{{N}^{-}}\] is also a strong field ligand, therefore, it also pairs up the unpaired electrons. \[_{28}Ni=[Ar]\,3{{d}^{8}}4{{s}^{2}}\], \[N{{i}^{2+}}=[Ar]\,3{{d}^{8}}\,\,4{{s}^{0}}\]
Due to \[S{{p}^{3}}\]-hybridisation, its shape is tetrahedral and due to absence of unpaired electron, it is diamagnetic in nature. II. In \[{{[Ni{{(CN)}_{4}}]}^{2-}}\], Ni is present as \[N{{i}^{2+}}\]-ion, since \[C{{N}^{-}}\] is also a strong field ligand, therefore, it also pairs up the unpaired electrons. \[_{28}Ni=[Ar]\,3{{d}^{8}}4{{s}^{2}}\], \[N{{i}^{2+}}=[Ar]\,3{{d}^{8}}\,\,4{{s}^{0}}\] 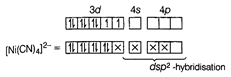 Due to \[ds{{p}^{2}}\] -hybridisation, its shape is square planar and due to absence of unpaired electron, it is diamagnetic in nature.
Due to \[ds{{p}^{2}}\] -hybridisation, its shape is square planar and due to absence of unpaired electron, it is diamagnetic in nature.
You need to login to perform this action.
You will be redirected in
3 sec
