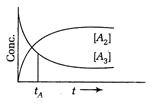
A) 75%
B) 50%
C) 40%
D) 30%
Correct Answer: C
Solution :
Let initial concentration is C of \[{{A}_{3}}\], then after sometime let it decrease by D, so \[{{A}_{2}}s\]concentration increases by \[\frac{3}{2}\times D\] due to stoichiometric ratios, when equal, \[C-D=\left( \frac{3}{2} \right)D=\left( \frac{2}{5} \right)C\] Hence, concentration of \[{{A}_{3}}\] reduced by \[\frac{2}{5}\] th or 40%.You need to login to perform this action.
You will be redirected in
3 sec
