A) \[{{[Zn{{(N{{H}_{3}})}_{4}}]}^{2+}}\]
B) \[{{(Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\]
C) \[{{[Ni{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
D) \[{{[Ni{{(CN)}_{4}}]}^{2-}}\]
Correct Answer: C
Solution :
\[{{[Zn{{(N{{H}_{3}})}_{4}}]}^{2+}}\] \[Z{{n}^{2+}}={{d}^{10}}\]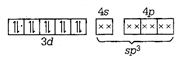 \[\therefore \,{{[Zn{{(N{{H}_{3}})}_{6}}]}^{3+}}\] is diamagnetic. \[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\] \[C{{o}^{3+}}={{d}^{8}}\]
\[\therefore \,{{[Zn{{(N{{H}_{3}})}_{6}}]}^{3+}}\] is diamagnetic. \[{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\] \[C{{o}^{3+}}={{d}^{8}}\] 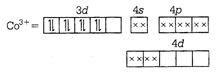 \[\therefore \,{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\] is also diamagnetic \[{{[Ni{{({{H}_{2}}O)}_{6}}]}^{2+}}\]
\[\therefore \,{{[Co{{(N{{H}_{3}})}_{6}}]}^{3+}}\] is also diamagnetic \[{{[Ni{{({{H}_{2}}O)}_{6}}]}^{2+}}\] 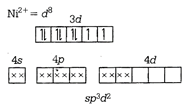 As \[{{H}_{2}}O\] is a weak field ligand, due to which pairing of electron did not occur. \[\therefore \] It is a paramagnetic in nature. \[Ni{{(CN)}_{4}}{{]}^{2-}}\] \[N{{i}^{2+}}={{d}^{8}}\]
As \[{{H}_{2}}O\] is a weak field ligand, due to which pairing of electron did not occur. \[\therefore \] It is a paramagnetic in nature. \[Ni{{(CN)}_{4}}{{]}^{2-}}\] \[N{{i}^{2+}}={{d}^{8}}\] You need to login to perform this action.
You will be redirected in
3 sec
