I. 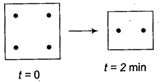 |
II. 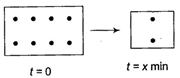 |
A) 2 min
B) 4 min
C) 6 min
D) 8 min
Correct Answer: C
Solution :
By set I, half-life is 2 min. In set-ll, number of moles have been doubled, thus half-life is also doubled, i.e., now it is 4 min. Thus, 8 mol change to 4 mol (half) in =4 min and 4 mol change to 2 mol (half) in =2 min. Thus, total time = 4 + 2 = 6 min.You need to login to perform this action.
You will be redirected in
3 sec
