A) \[{{H}_{2}}O\]
B)
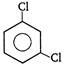
C) \[\underset{H-C-Cl}{\overset{H-C-Cl}{\mathop{||}}}\,\]
D) \[C{{O}_{2}}\]
Correct Answer: D
Solution :
The dipole moment of\[C{{O}_{2}}\]is zero due to its linear structure. \[O\overset{\leftarrow }{\mathop{=}}\,C\overset{\to }{\mathop{=}}\,O\] The individual bond moments of two bonds are equal in magnitude but in opposite direction hence, the two bond moments cancel to each other.You need to login to perform this action.
You will be redirected in
3 sec
